Developmental Neurobiology
Cristina Pujades

Group website
Research Outline
Our goal is to understand how spatiotemporally coordinated cell progenitor specification and differentiation occur during morphogenesis to construct a functional brain. While incorporating time as a missing-yet-crucial factor, we want to provide a holistic view of how cell fate decisions are taken in the brain in order to gain biological insight into i) how tissue growth is intertwined with cell proliferation and differentiation; ii) how the heterogeneity of neural progenitors is generated, and iii) how individual progenitors behave during patterning and morphogenesis.
We focus on the embryonic brainstem, which controls life-sustaining functions and is extremely conserved in vertebrates, using cutting-edge complementary approaches such as CRISPR-Cas9 genome editing, high-resolution 4D-imaging paired with cell tracking tools, and analyses of gene regulatory landscapes.
Research Lines
1. Determining how cell diversity is generated in the developing hindbrain, by reconstructing cell lineages of the distinct hindbrain territories over time and unveiling the molecular mechanisms responsible for cell fates/states transition.
2. Deciphering the contribution of specific progenitor pools to specific neural circuits, by studying the spatiotemporal growth of the neuronal differentiation domain, and determining the contribution of different progenitor pools to the distinct functional neuronal populations by intersectional genetic tools.
3. Elucidating how the balance between cell proliferation and differentiation is regulated, by addressing whether different progenitor pools display distinct modes of growth using multicolor lineage tracing.
We use the zebrafish embryo as a model system since allows us to combine high-resolution in vivo imaging with genetics and genome-editing tools. With this combination of approaches and incorporating time as the missing-yet-crucial factor, we want to elucidate how gene regulatory networks operate during development, tissue degeneration, and regeneration.
Team during 2019-20
PhD students: Ivan Belzunce, Carla Belmonte-Mateos, Carolyn Engel-Pizcueta, Matthias Blanc
Postdocs: Covadonga Fernández-Hevia
Technicians: Laia Subirana, Marta Linares
Selected publications
-
Pujades, C (2020). The multiple functions of hindbrain boundary cells: tinkering boundaries? Invited review for the Special Issue in Cell Sorting at Embryonic Boundaries. Editor: François Fagotto. Sem in Cell and Dev Biol May 21:S1084-9521(19)30238-1; DOI: 10.1016/j.semcdb.2020.05.002
-
Belzunce I, Belmonte-Mateos C, Pujades C (2020). The interplay of atoh1 genes in the lower rhombic lip during hindbrain morphogenesis. PLoS ONE 3; 15(2): e0228225; DOI: 10.1371/journal.pone.0228225
*Article chosen as Research Highlight by PLoS ONE
-
Voltes, A, Hevia, CF, Engel-Pizcueta, C, Dingare, C, Calzolari, S, Terriente, J, Norden, C, Lecaudey, S, Pujades, C (2019). Yap/Taz-TEAD activity links mechanical cues to specific to cell progenitor behavior during hindbrain segmentation, Development Jul 22; 146(14): dev176735; DOI: 10.1242/dev.176735
*Article chosen as Research Highlight by Development, The Company of Biologists
-
Pant DC, et al.…Pujades C, Fatemi A, Boespflug-Tanguy O, Pujol A (2019). Loss of the sphingolipid desaturase DEGS1 causes hypomyelinating leukodystrophy. J Clin Invest Mar 1;129(3):1240-1256; DOI: 10.1172/JCI123959
Other relevant information
2019: Invited visiting scientist at Janelia HHMI Research Campus, Ashburn, US
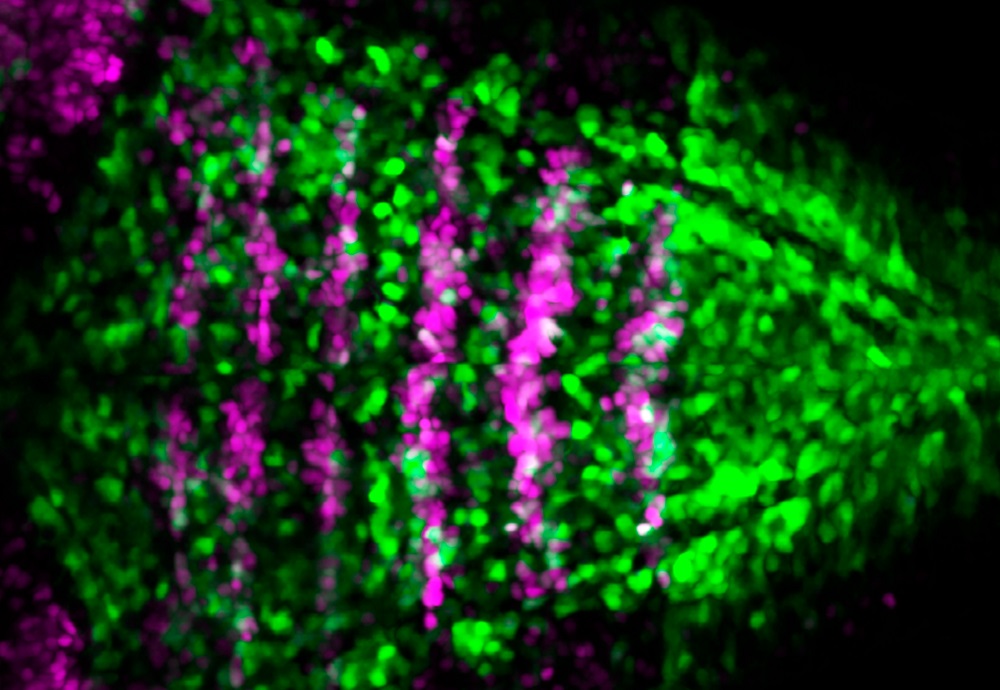
Notch-activity is restricted to specific territories in the embryonic hindbrain. Double transgenic Tg[BCP:H2AmCherry; tp1:d2GFP] zebrafish embryos allow us to see that Notch-active cells (in green) dispay a salt-and-pepper pattern, and boundary cells (in magenta) have little Notch-activity at this embryonic stage [Carolyn Engel-Pizcueta]
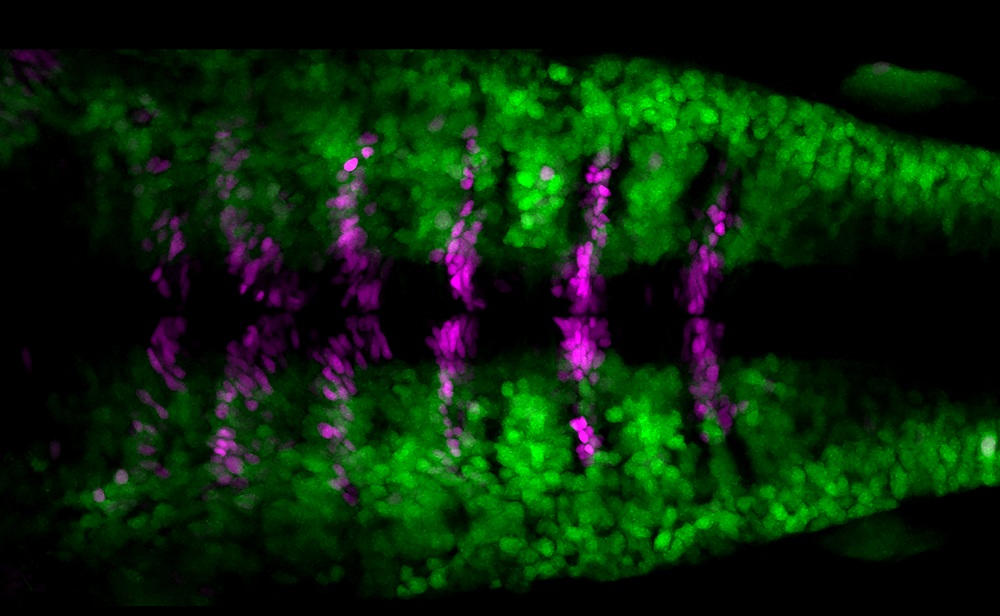
Hindbrain boundary cells do no undergo neurogenesis at early stages of embryonic development. Double transgenic Tg[BCP:H2AmCherry; HuC:GFP] zebrafish embryos show that a specific cell population called boundary cells (in magenta) do not display the pan-neuronal differentiation marker HuC (in green), whereas the neighboring cells do. Dorsal view with anterior to the left [Covadonga F Hevia].
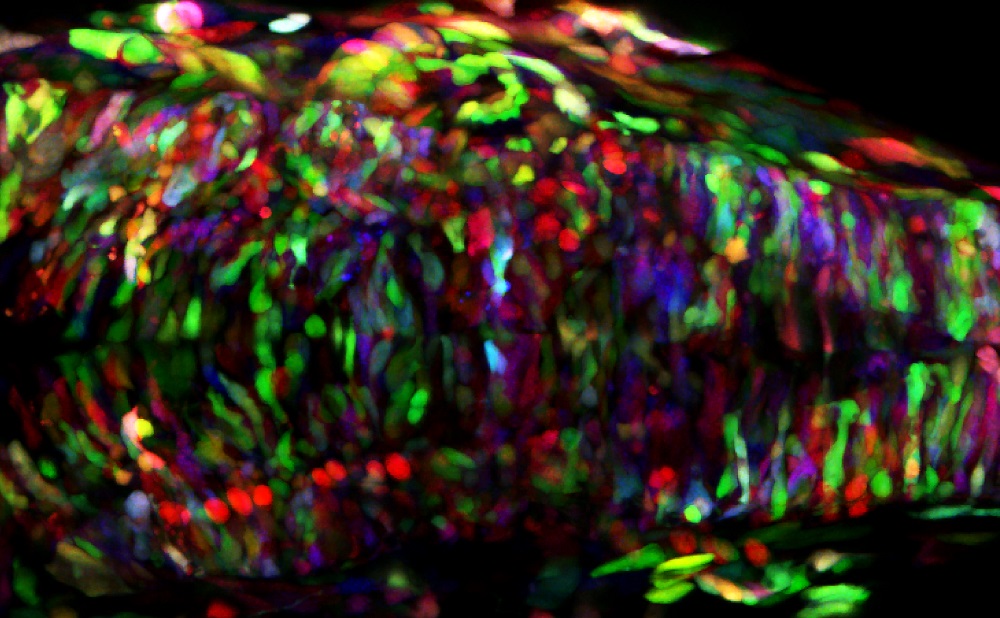
Multicolor lineage tracing using the ZEBRABOW technique. Zebrabow embryo in which the different cell lineages are genetically labelled in different colors. Colors are inherited equally among daughter cells and remain stable throughout embryonic and larval stages [Carolyn Engel-Pizcueta].
