CRG/UPF Advanced Light Microscopy Unit (ALMU)
Timo Zimmermann
Website
Research Outline
The Advanced Light Microscopy Unit (ALMU) serves as a core facility for high-end light microscopy for PRBB researchers. A range of instruments with unique capabilities fully covers the spectrum of advanced imaging applications from thick tissue reconstruction to fast in-vivo imaging to the sensitive detection of very faint signals of single molecules. The staff of the facility provides advice in the initial experiment planning, training of the researchers on the instruments and assistance with the subsequent data analysis.
The facility usage is around 15000 hours per year booked by around 200 users from more than 60 research groups.
Services
It is the aim of the facility to provide a link for the biological questions of researchers to the full capabilities of advanced light microscopy at the organismic, cellular and molecular level. Methods available in the facility include super-resolution microscopy by stimulated emission depletion (STED) and localization based methods like Stochastic Optical Reconstruction Microscopy (STORM) and Ground State Depletion Imaging Microscopy (GSDIM), optical sectioning (single photon and multi-photon microscopy), spectral imaging, in-vivo time-lapse imaging, Total Internal Reflection Fluorescence (TIRF) Microscopy and methods for the study of molecular properties and interactions like Fluorescence Correlation Spectroscopy (FCS), Fluorescence Lifetime Imaging Microscopy (FLIM), Fluorescence Resonance Energy Transfer (FRET) detection, Fluorescence Recovery after Photobleaching (FRAP) and confocal and widefield screening microscopy. Additionally, dedicated software packages for data visualization and analysis are available for 3D rendering, particle tracking and image analysis.
Team during 2019-20
- Technicians: Raquel Garcia (CRG), Arrate Mallabiabarrena (CRG), Xavier Sanjuan (UPF), Raúl Gómez (CRG)
Selected publications 2019-20
Zimmermann T. (2019) Photobleaching and Sensitized Emission-Based Methods for the Detection of Förster Resonance Energy Transfer. In: Rebollo E., Bosch M. (eds) Computer Optimized Microscopy. Methods in Molecular Biology, vol 2040. Humana, New York, NY. https://doi.org/10.1007/978-1-4939-9686-5_12
Dumbović G, Sanjuan X, Perucho M, Forcales SV (2020). Stimulated emission depletion (STED) super-resolution imaging of RNA- and protein-containing domains in fixed cells Methods. Apr 30:S1046-2023(19)30323-8. doi: 10.1016/j.ymeth.2020.04.009. Online ahead of print.PMID: 32360441 (see figure)
Other relevant information 2019-20
In 2019 ALMU hosted together with EMBL Barcelona’s Mesoscopic Imaging Facility open technology workshops showcasing the latest technologies of the companies Leica, Nikon and Andor in the Leica “See the Hidden” event and the Andor Imaging Academy. It also organized the demonstration and user testing of a new microscope system for holotomography by Nanolive.
In 2020 ALMU was selected by Leica to perform a beta-test of Stellaris, a new confocal platform. The test was performed partially during Covid-19 lockdown, nevertheless, the facility staff could provide relevant feedback to Leica.
The unit participated in the execution of BIST Winter School on Microscopy and Imaging Sciences 2019 and 2020 which took place from January to February for the BIST Master Course of Multidisciplinary Research in Experimental Sciences.
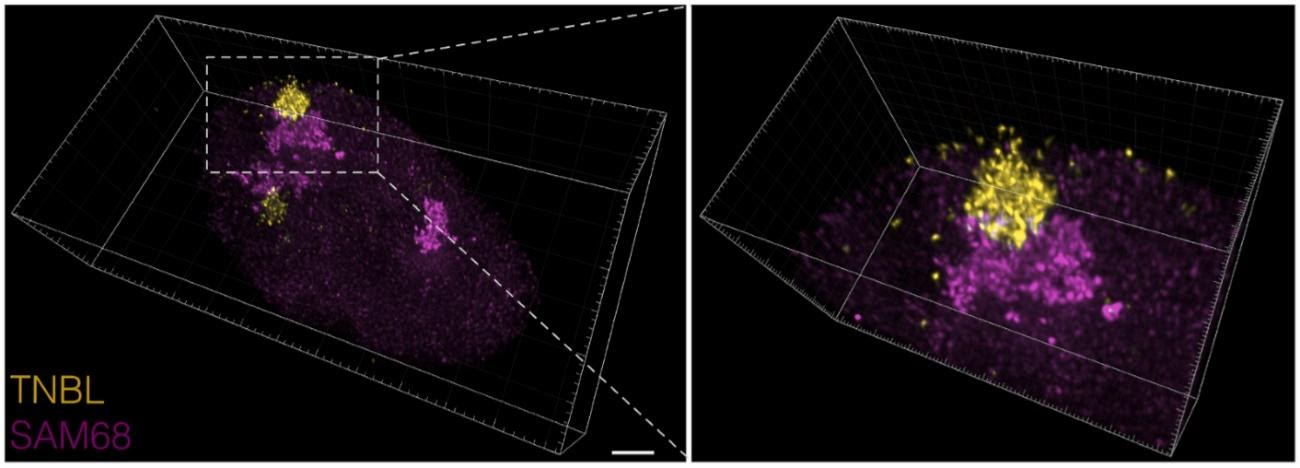
3D visualization RNA- and protein-containing nuclear domains obtained by STED imaging of an entire cell volume. Z-stacks were deconvolved with Huygens deconvolution software and visualized in Imaris (version 8.3). On the left, the entire cell nucleus is shown, scale bar: 2 μm; on the right a zoom of the adjacent structures. RNA clusters shown in yellow, protein bodies in magenta. (Extracted from Dumbovic et al (2020)).
