Cancer Biology
Ana Janic
Group website
Research Outline
The tumour suppressor gene p53 is mutated in half of the human cancers, and there is still extensive morbidity and mortality associated with cancers bearing p53 mutations. Given the difficulties in developing strategies for targeting wild-type or mutant p53, further understanding of its basic biology is required for successful clinical translation. Recent studies, including ours, have challenged the previously understood model of how the p53 gene is involved in tumour suppression. We found that several p53 activated target genes implicated in DNA repair have critical functions in suppressing blood cancer development. Based on this observation, we hypothesise that the coordination of DNA damage repair is the most critical mechanism by which p53 suppresses tumour development. In line with this hypothesis our laboratories current research focuses on answering the following questions i) how p53 controls a DNA repair–coordinated program to protect tumorigenesis; ii) how tissue specificity controls which p53-regulated DNA repair effectors are crucial for tumour suppression and iii) how we could use p53-dependent DNA repair signalling therapeutically to kill tumour cells.
Research Lines
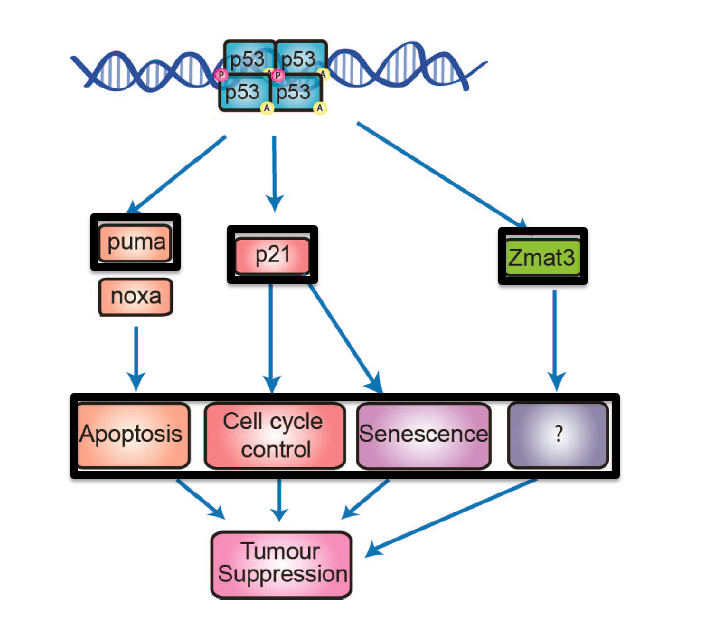
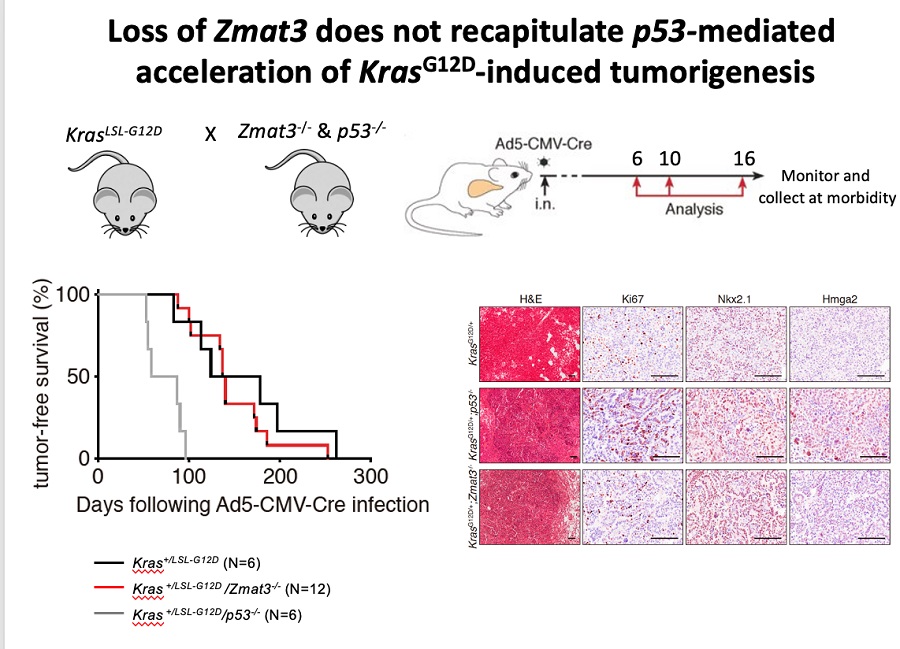
Our laboratories current research focuses on answering following questions i) how p53 controls a DNA repair–coordinated program to protect tumorigenesis; ii) how tissue specificity controls which p53-regulated DNA repair effectors are crucial for tumor suppression and iii) how we could use p53-dependent DNA repair signaling therapeutically to kill tumor cells. Additionally, our laboratory is studying p53 target genes, which play roles in the process of malignant transformation. One such target, ZMAT3 is a double-stranded RNA binding zinc finger protein whose function in tumorigenesis is relatively unknown. In our laboratory we are investigating the contribution of ZMAT3 to tumorigenesis in vivo by using unique Zmat3 knockout mice. Together, our research program is dedicated to understanding the complex p53 tumour suppression transcriptional network in different tissues and in the context of distinct oncogenic drivers.
Team during 2019-20
- PhD students: Etna Abad, Julia Urgel.
- Technician: Laia Subirana
Selected publications
- Best SA, Vandenberg CJ, Abad E, Whitehead L, Guiu L, Ding S, Brennan MS, Strasser A, Herold MJ, Sutherland K*, JANIC A*. (2020) Consequences of ZMAT3 loss in c-MYC- and mutant KRAS-driven tumorigenesis. Cell Death & Disease. doi:10.1038/s41419-020-03066-9 *Joint senior authors.