PharmacoInformatics
Manuel Pastor

Group website
Research Outline
Our research is focused in the development and application of computational methods and software to pharmaceutical development. Recently we focused our attention on the safety assessment of drugs candidates, where the use of computational methods has many advantages with respect to classical methodologies based on the observation of in vivo endpoints. Our group has developed numerous predictive models in this field and produced software which can be used to deploy models in industrial environments. Other methodological contributions were multiscale methods for the prediction of drug-induced QT prolongation or robust methods for assessing the reliability of toxicity predictions.
Current Projects
- iPiE
The iPiE project aims to utilize information from toxicological studies, pharmacological mode of action and in silico models to support intelligence-based environmental testing of pharmaceuticals in development and to prioritize legacy pharmaceuticals for targeted environmental risk assessment and/or environmental monitoring.
- eTRANSAFE
This project will compile toxicological data generated by the pharmaceutical industry at different stages, as well as pharmacovigilance data, and build a sophisticated database allowing to exploit this information using advanced query and visualization software. This information will be further exploited by developing predictive models, exploring the translability of the preclinical data and identifying biomarkers.
- EU-ToxRisk
The project EU-ToxRisk aims to drive a paradigm shift in toxicological testing away from animal testing towards a toxicological assessment based on human cell responses and mechanistic understanding of chemical adverse effects. The ultimate goal is to deliver testing strategies to enable reliable, animal-free hazard and risk assessment of chemicals.
Team during 2017-18
- Postdocs and senior researchers: Nuria B. Centeno, Ismael Zamora,
Elisabet Gregori, José Carlos Tamayo .
-
PhD students: Kevin Pinto.
-
Technicians: Gabriel Stela and Ignacio Pasamontes.
Selected publications 2017-18
- Pastor M, Quintana J, Sanz F (2018) Development of an Infrastructure for the Prediction of Biological Endpoints in Industrial Environments. Lessons Learned at the eTOX Project. Front Pharmacol. 9:1147.
- Romero L, Cano J, Gomis-Tena J, Trenor B, Sanz F, Pastor M, Saiz J (2018) In Silico QT and APD Prolongation Assay for Early Screening of Drug-Induced Proarrhythmic Risk. J Chem Inf Mod. 58(4)867-78.
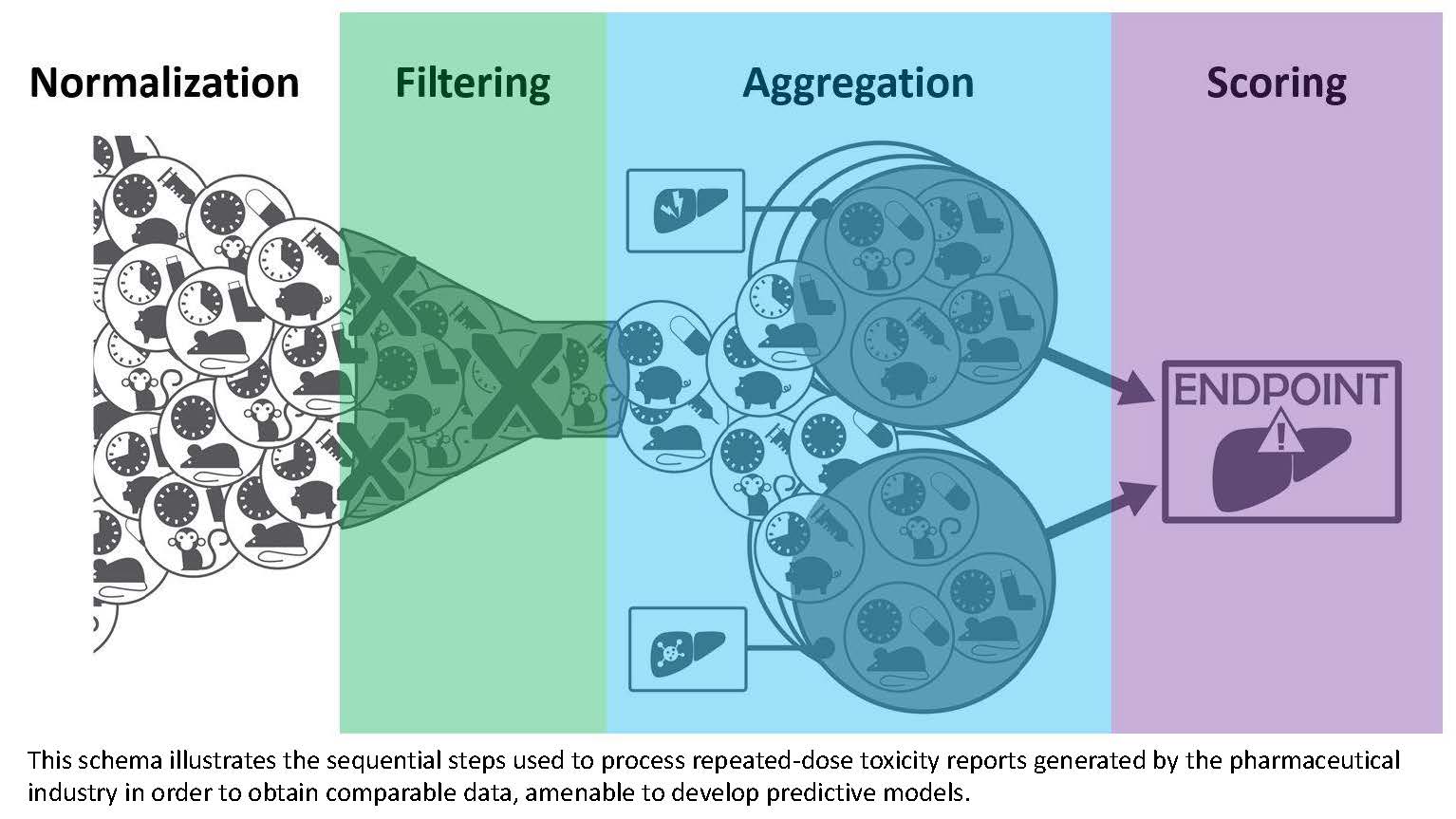
- López-Massaguer O, Pinto-Gil K, Sanz F, Amberg A, Anger LT, Stolte M, Ravagli C, Marc P, Pastor M (2018) Generating modelling data from repeat-dose toxicity reports Tox Sci. 162(1)287-300.
- López-Massaguer O, Sanz F, Pastor M (2018) An automated tool for obtaining QSAR-ready series of compounds using semantic web technologies Bioinformatics. 34(1)131–3.
- Sanz F, Pognan F, Steger-Hartmann T, Díaz C and eTOX (including Pastor M) (2017) Legacy data sharing to improve drug safety assessment: the eTOX project Nat Rev Drug Discov. 16:811–2.