Research interests
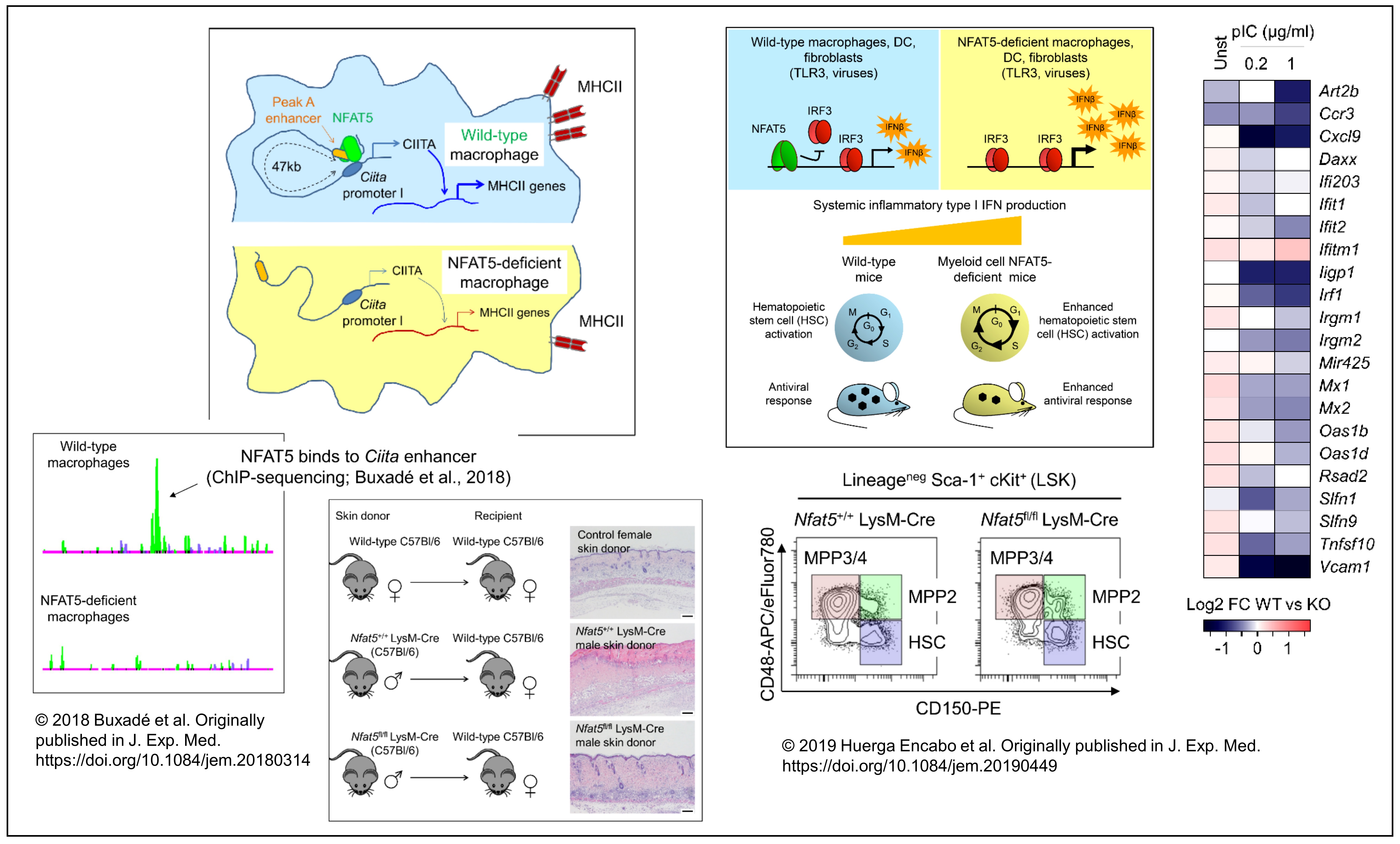
Our group’s main interest is to identify mechanisms that enable immune cells to modulate complex inflammatory responses in antipathogen defense and diseases such as cancer and metabolic disorders. Our work has focused on NFAT5, a Rel-like transcription regulator that we contributed to identify two decades ago. Our group opened up the field of NFAT5 in immune responses nine years ago by uncovering primary mechanisms by which NFAT5 controls innate and adaptive immune responses (J. Experimental Medicine, 2012; Proc Natl Acad Sci USA, 2013). In recent works we have shown that NFAT5 can modulate chromatin configuration, oppose repressive histone marks, and cooperate or compete with other transcription factors. These mechanisms can boost or restrain key immune functions, such as the control of inflammatory antiviral responses to preserve stem cell quiescence, regulation of antitumor innate immunity, or graft rejection (J. Experimental Medicine 2018 and 2020, J. Immunology, 2018 and 2021). These findings have given rise to various current projects, which address the connection of inflammation with stem cell potential, how inflammation adjusts immune cell metabolism, and explore directions for improving antitumor immunotherapies.