Characterization of 7q11.23 reciprocal aneusomy syndromes: from patients to functional pathways (and back)
 Williams-Beuren Syndrome (WBS) and 7q11.23 microduplication syndrome (7dup) are two rare developmental disorders caused by a deletion or duplication, respectively, of a chromosome region at 7q11.23. Patients present opposite craniofacial characteristic and cardiac alterations, and both have intellectual disability. These rearrangements expand 1.55-1.83 Mb and include 26-28 genes.
Williams-Beuren Syndrome (WBS) and 7q11.23 microduplication syndrome (7dup) are two rare developmental disorders caused by a deletion or duplication, respectively, of a chromosome region at 7q11.23. Patients present opposite craniofacial characteristic and cardiac alterations, and both have intellectual disability. These rearrangements expand 1.55-1.83 Mb and include 26-28 genes.
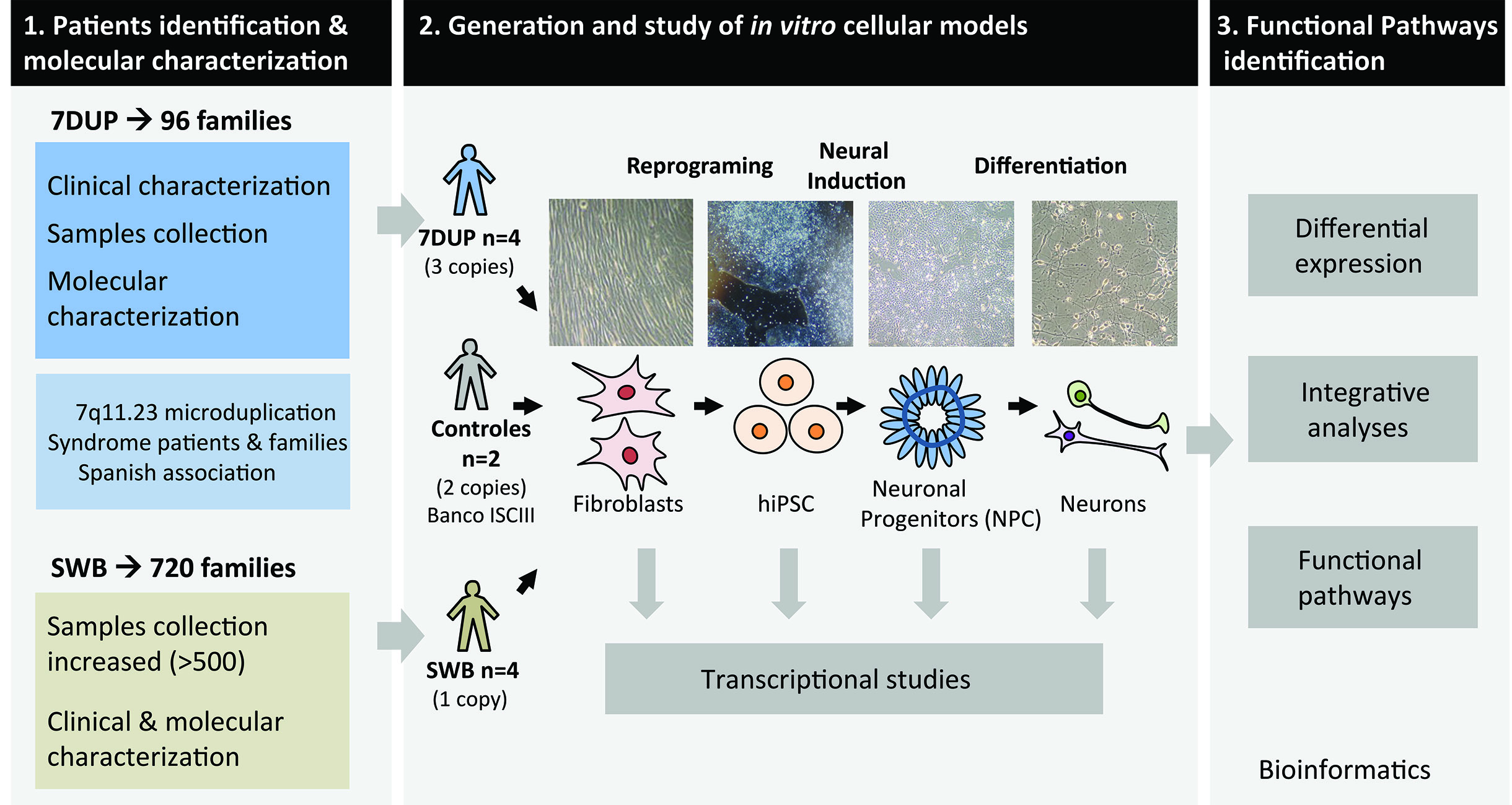
To better understand the pathophysiological mechanisms disrupted in patients carrying 7q11.23 alterations, we proposed a combined experimental and systems biology analytical approach with the following specific aims: (1) Identify and characterize patients with 7q11.23 CNVs; (2) Create reciprocal genetic models of 7q11.23 CNVs by generating induced pluripotent stem cells derived from patients and identify transcriptional alterations in differentiated neural precursors and mature neurons; and (3) Identify key functional pathways commonly and/or symmetrically disturbed across patient-specific neurons of 7q11.23 CNVs.
In this project, we have characterized at molecular level large collections of patients with variations in the number of copies of 7q11.23. In particular, we have demonstrated that the mechanisms leading to 7q11.23 microduplications are the reciprocal of the deletion events. In addition, we have identified recombination hotspots in a repetitive region that flanks the deleted/duplicated genes.
In the second aim of this project, we have created reciprocal genetic models of 7q11.23 copy number variants by generating patient-derived induced Pluripotent Stem Cells (iPSC) that were differentiated to neural precursors and differentiated neurons. We then studied the changes in the transcriptome of these in vitro models of brain to investigate the genes that are altered by these alterations at 7q11.23.
Finally, we used a Systems Biology approach integrating multiple sources of biological evidence to identify genes deregulated and pathways altered in these disorders. We identified multiple genes differentially expressed in specific types of neurons that warrant further investigation.
These cellular models are now a very valuable resource for future studies for 7q11.23 syndromes and associated brain disorders as autism spectrum disorders. Further studies will include the analyses of morphology and functionality of these neurons, and assays or in vitro drug testing.
FUNDING
- Marie Skłodowska-Curie actions 2014, Individual Fellowship, Horizon 2020
- “Todos Somos Raros, Todos Somos Únicos”
LINKS
Patients support group:
Asociación Nacional de Afectados por la Duplicación Cromosómica 7q11.23
http://www.7q1123-duplicados.org
Todos Somos Raros
http://www.todossomosraros.es/
Marie Skłodowska-Curie actions
https://ec.europa.eu/programmes/horizon2020/en/h2020-section/marie-sklodowska-curie-actions

