Research
CURRENT PROJECTS

Morphogenesis
The inner ear is one of the most complex three-dimensinal organs of our head, however it is still little understood how cells organize during development to generate this complex organ. We are studying several morphogenetic events to understand the interaction between cell polarity, cell remodeling, migration and cytoskeleton rearrangements with mechanical properties and signalling cues.
- Sensory neuroblast delamination and migration
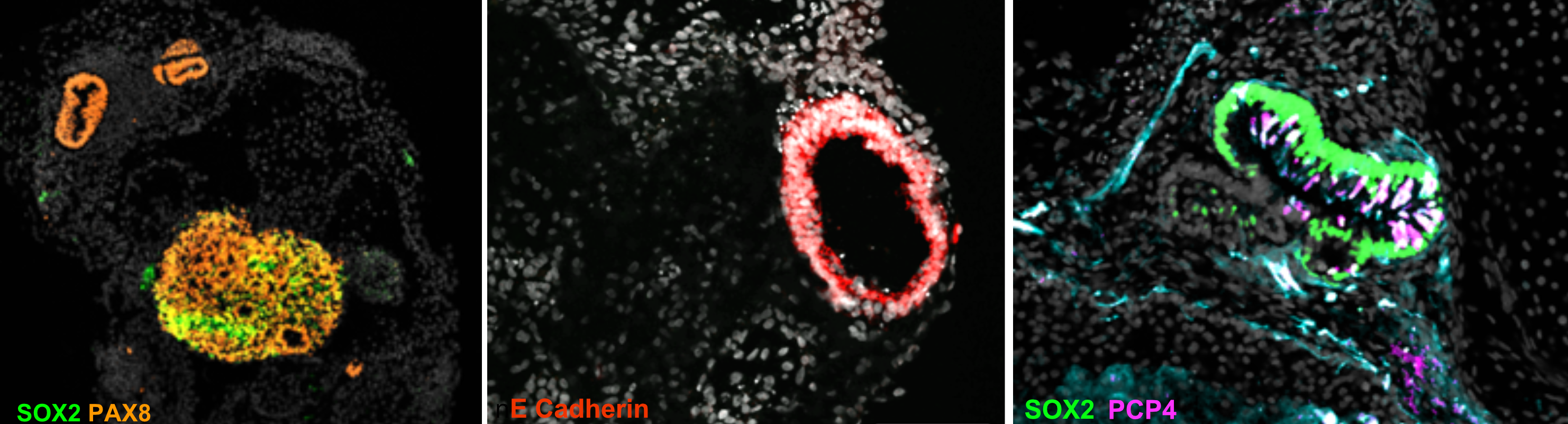
Neural specification begins within the otic placode by the activation of neurog1 in otic stem cells in the most anterior-lateral domain (Abello et al, 2007). The epithelial neurogenic progenitors divide, and then delaminate out of the epithelium and coalesce into the statoacoustic ganglion (SAG). Lillte is known on the migratory dynamics and the spatial organization of neuroblasts within the SAG.
By single-cell labelling of neuroblasts we are following their migratory paths, describing the axon patterns and innervation dynamics in zebrafish.
How neuroblasts migrate and colesce to organize into the 3D SAG? Do they communicate to each other to follow a specific path? How specific innervation patterns are established?
- Otic placode formation
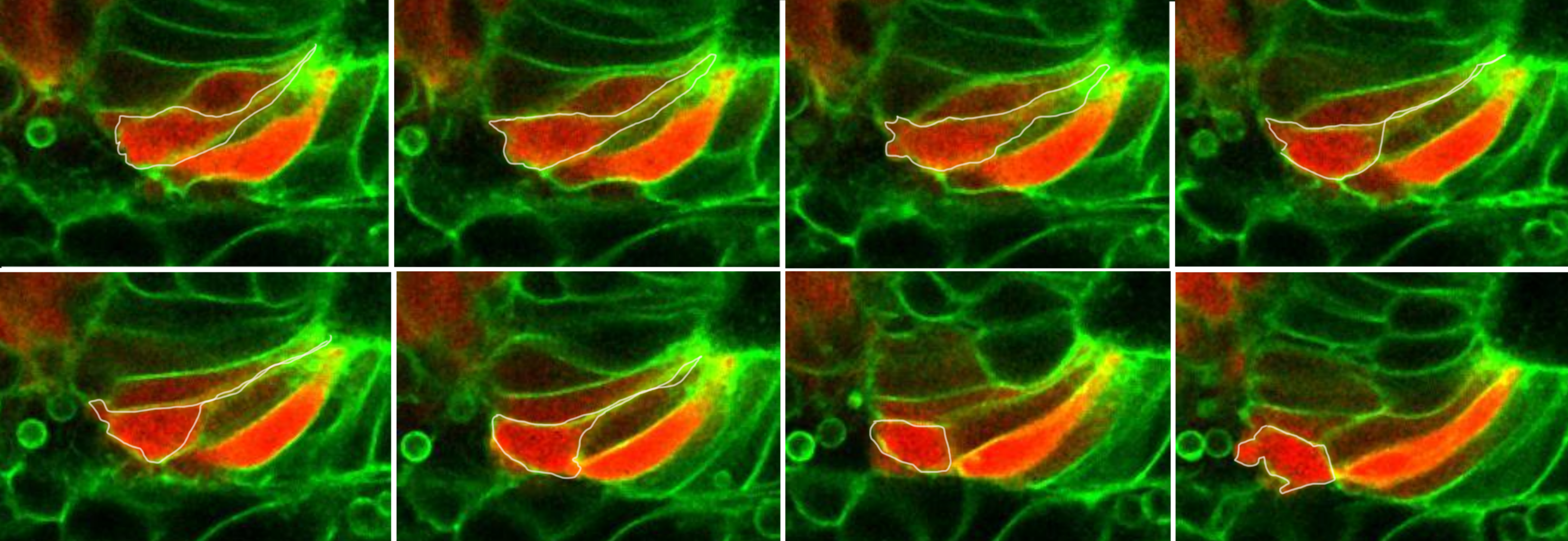
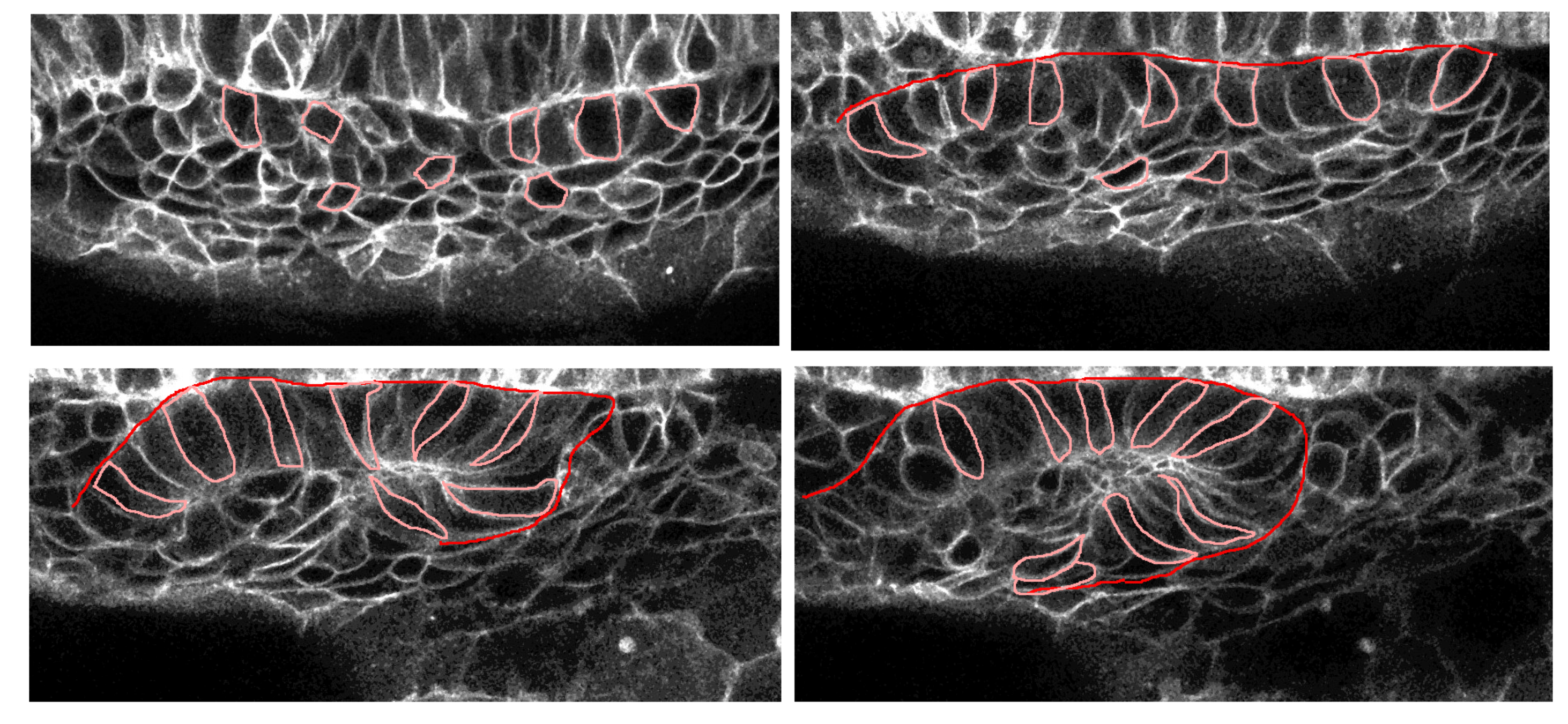
Otic precursors are initially not organized, cells have arounded shape and progressively orient and elongate to form a ordered epithelium. The sequence of events involving the establishment of apical polarity, microtubule orientation, actomyosion activation and rosette formation is unknown. We aim to understand the cellular remodelling events during placode formation and the biomechanics driving the formation of an ellipsoidal placode.

Neurovascular Development
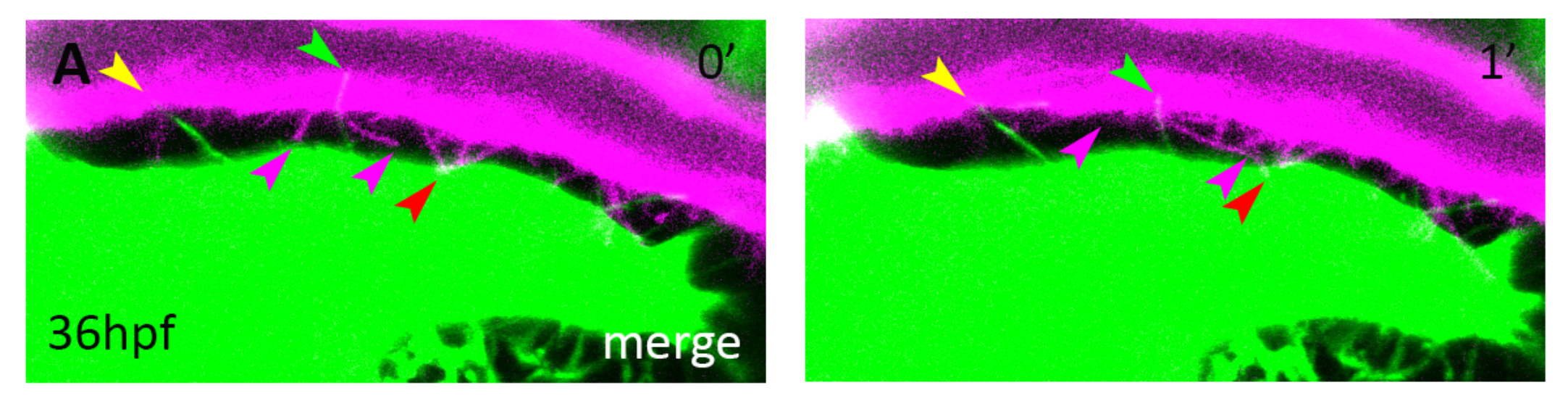
Most attention to understand SAG growth has focused into signals from the ganglion itself. we have created an anatomical map of the network of blood veseels covering the SAG (Taberner et al. 2017). Blood vessels and nerve fibers course through the body in complicated patters, often aligned one to the other. In recent years, it has been put in evidence that signaling interaction "vessels to nerves" and "nerves to vessels" enables their growth, survival, differentiation and migration in the subventricular zone and other organs.
We have shown that cranial vasculature is required for two independent roles in neurogenesis. An early role mediated by cytonemes and Dll4-Notch signalling, regulates neuroblast proliferation. Another role mediated by blood flow promotes the swith from hypoxic metabolism to OxPhos in neuroblast and induces their differentiation (Taberner et al., Cell Reports 2020).

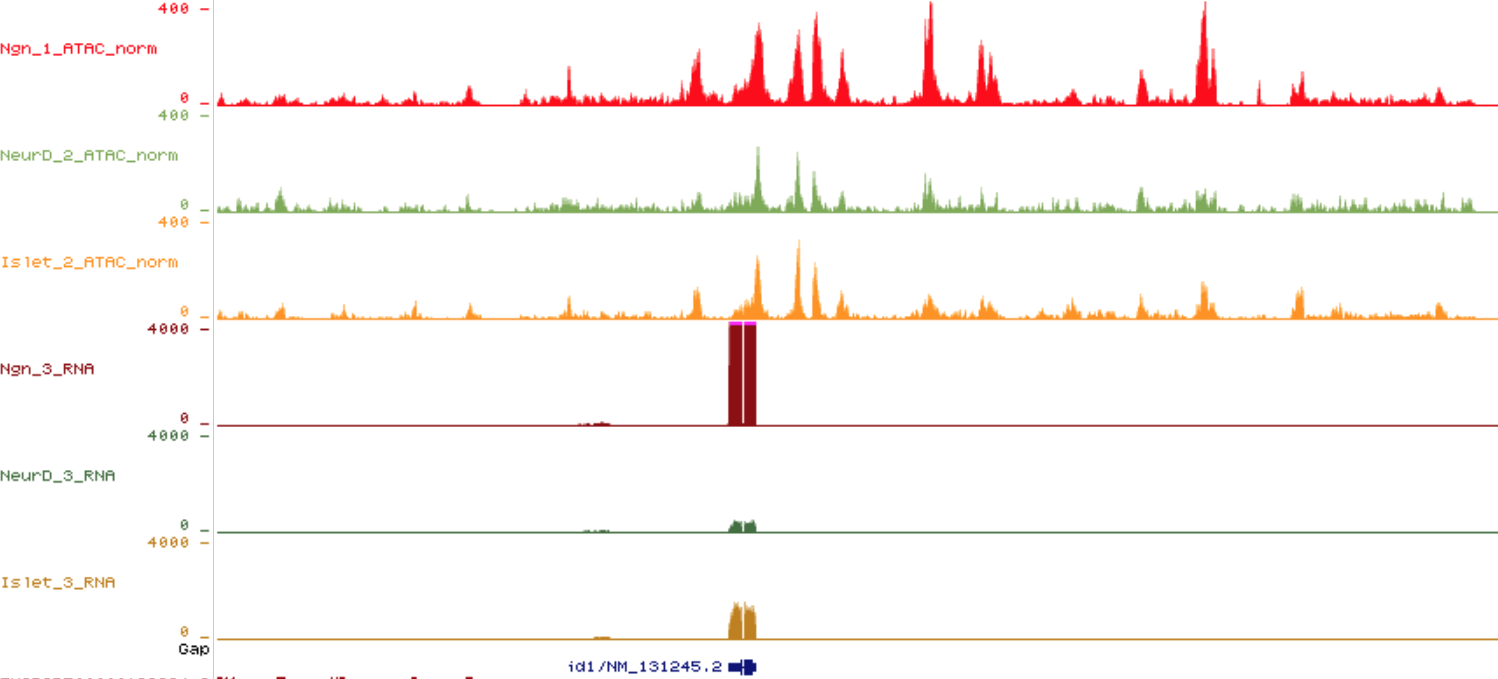
Transcription factors and chromatin dynamics in otic neuron identity
The identification of TF driving neuronal differentiation and subtype sensory identities is crucial for regenerative medicine therapies and for the understanding the basis of sensory diseases. Compared to the central nervous system and the development of hair cells, very little is known of how the transition from inner ear neuronal precursors to differentiated neurons is achieved at the molecular level. What makes an otic sensory neuron different from a nociceptor or chemosensory neuron?
We aim at identifying which TFs are key in otic sensory neuronal identity and maintaning their terminal features, together with the analysis of the chromatin remodelling events regulating the difference between distict sensory neuronal subtypes.
Inner ear organoids for studying mutations causing hearing loss
Several mutations either in the coding regions or regulatory elements have been linked to hearing loss, vertigo or neuropathies. In some instances, the sensory disease is not due to a malfunctioning protein but to a reduction/increased of expression or epigenetic modifications of the affected loci. Those mutations might be rescued by modification of the expression levels using the crispr system. We are testing the use of the cripr system to revert regulatory mutations using human inner ear organoids.