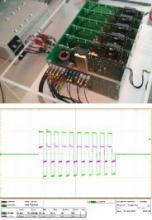
This novel device is able to generate high voltage pulses and current, with better performance than current available generators.
BACKGROUND
The use of the technique of irreversible electroporation (IRE) requires the development of high-voltage and high-current generators showing also a high performance and controllability. In clinical applications, it is also required these generators to be compact, light, reliable and with high performance regarding electrical safety. In nowadays medical applications, available standard unipolar pulse generators are limited in voltage and maximum currents, showing severe constrictions regarding duration of pulses, configuration and requiring needle-type electrodes, which hinders their application in irreversible electroporation of tumor tissues.
THE TECHNOLOGY
This novel device is able to generate high voltage pulses (tested with up to 12 kV peak to peak) and current (tested up to 400 A peak to peak), with better performance than current available generators. This means that ablation volumes will be higher than those achieved currently. It also allows better flexibility in the output voltage: bipolar voltage, pulse width (from 1 µs) and number of pulses and pulse trains, which are fully configurable. This has positive implications for clinical treatment.
ADVANTAGES
- Higher voltage and current ratings, enabling larger treated regions in the same procedure. Planar electrodes can be used for improved, controlled and homogeneous treatment.
- It is possible to design operation protocols where short time and fast electric pulses are applied. Therefore, treatment times are significantly shortened.
- The device is more compact and lighter than current ones, because no low- frequency transformer is required.
- Power supply using batteries, leading to improved safety and electric isolation. Compliance with EMC regulations and approval process is simplified.
- Since the control architecture is based on a FPGA, future advanced functions can be implemented such as ECG synchronization, treatments automation.
STATE OF DEVELOPMENT
Fully functional prototype.
Compelling results in vegetal and in living animal tissue.
INTELLECTUAL PROPERTY
Patent application filed.
Technology ownership shared among Universidad de Zaragoza (66%) and Universitat Pompeu Fabra (34%).
MARKET OPPORTUNITY
Global ablation technologies market expected CAGR of 9.6% from 2014 to 2019. Electroporation market expected CAGR of +10% from 2010 to 2016.
COMMERCIAL OPPORTUNITY
Technology available for licensing with technical cooperation
CONTACT
Technology Transfer Unit
(+34) 93 542 2187
[email protected]
KEYWORDS
Electroporation, tumor ablation.
Ref: TEC-0129/P-0033
Fact Sheet