Scientists reveal for the first time the details of protein association at atomic level
In just one year, an international team of researchers has succeeded in simulating the association and dissociation of proteins in atomic detail. If they had used a supercomputer, this process would have taken 10,000 years.
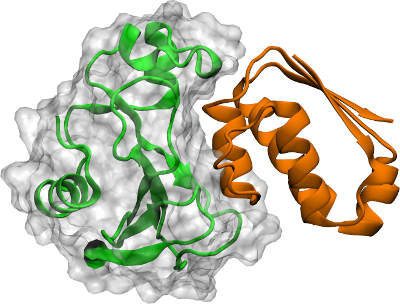
Scientists from Freie Universität Berlin (FU Berlin) and Pompeu Fabra University in Barcelona have, for the first time, simulated the association and dissociation of protein molecules at atomic level. The results, published in the scientific journal Nature Chemistry, were validated with experimental data.
Almost everything that happens in our body is driven by proteins that find and bind to each other – to transmit information or build up living matter. Proteins and other molecules move around in our cells, and they can associate to and dissociate from each other. Our muscles, for example, consist of proteins that have associated to each other so as to form long fibres. If we move our muscles, the muscle fibres must slide relative to one another, and they do so by continuously forming protein-protein contacts. One fibre pulls the other to exert the muscular force, and then the protein-protein pairs dissociate again, in order to prepare for the next step.
A costly process in terms of computation and temporarily
As yet, nobody has been able in atomic detail to observe how proteins associate. The problem is that proteins are extremely small, about a billionth of a metre, and their structures fluctuate a lot, making this process invisible to microscopes or other experimental techniques.
The research groups of Frank Noé, at FU Berlin, and Gianni De Fabritiis, ICREA research professor at UPF, have now collaborated to produce what is the first atomic-detail computer simulation of the process of protein-protein association and dissociation. The main challenge was that atomic-detail molecular dynamics are incredibly expensive to simulate. The interactions of about 100,000 atoms need to be computed, and the force felt by every atom is evaluated. Then, the simulation moves every atom by a small distance in the direction of the force while it advances the simulated time by a femtosecond – a tiny fraction of a second. Each such step is computationally expensive, but a billion of such steps need to be performed in order to simulate the movement of one hour that is the typical time the proteins stay bound before they dissociate again.
The simulation in molecular dynamics of protein association was thought to be impossible to perform at computational level due to the amount of time needed to take samples of the biochemical process: even using a supercomputer, it is estimated that the process would have required 10,000 years to complete. “This is the type of high-risk project that is very difficult to get funding for, because when we started, no-one would believe that it’s even possible”, says Noé.
Key to the success of the Berlin-Barcelona team was the combination of several new technologies that enabled a “divide and conquer” approach to the problem. GPUGRID, a distributed network developed by De Fabritiis’ group, was employed to store computing time on graphic processing units (GPUs) from the company Nvidia by volunteers from around the globe. Thousands of short simulations were conducted in that way, coordinated by a novel machine learning algorithm so that the overall protein association process could be simulated within one year instead of having to wait 10,000 years. Markov modelling, a method pioneered by Noé and colleagues, was used to combine the many short simulations to an overall dynamical model that describes protein association and dissociation in full detail.
“This was clearly a risky but important proof of principle and we are happy that we managed to show that the simulations are able to capture associations between proteins”, says De Fabritiis, head of the Computational Biophysics group at the Research Programme on Biomedical Informatics (GRIB), a joint programme between Pompeu Fabra University and the Hospital del Mar Medical Research Institute (IMIM).
Nuria Plattner, the lead author of the study, explains the implication of the results: “Our simulations reveal many previously unknown details on how proteins find and bind to each other. But the most valuable result of our study is the demonstration that studying protein-protein association in atomic detail is possible”. This achievement opens the door to understanding the details of viral infections, the inner workings of the immune system, and many other problems of biomedical or biotechnological relevance.
The project was supported by the European Research Council (ERC), the German Research Foundation (DFG), the Spanish Research Council (CSIC) and the Einstein Foundation Berlin.
Reference work: Nuria Plattner, Stefan Doerr, Gianni De Fabritiis, Frank Noé. Complete protein–protein association kinetics in atomic detail revealed by molecular dynamics simulations and Markov modelling. Nature Chemistry, June 2017. DOI: 10.1038/NCHEM.2785
