Understanding the biology and possible biological treatments of cartilage for osteoarthritis of the knee, using computational modelling
Understanding the biology and possible biological treatments of cartilage for osteoarthritis of the knee, using computational modelling
Understanding the biology and possible biological treatments of cartilage for osteoarthritis of the knee, using computational modelling
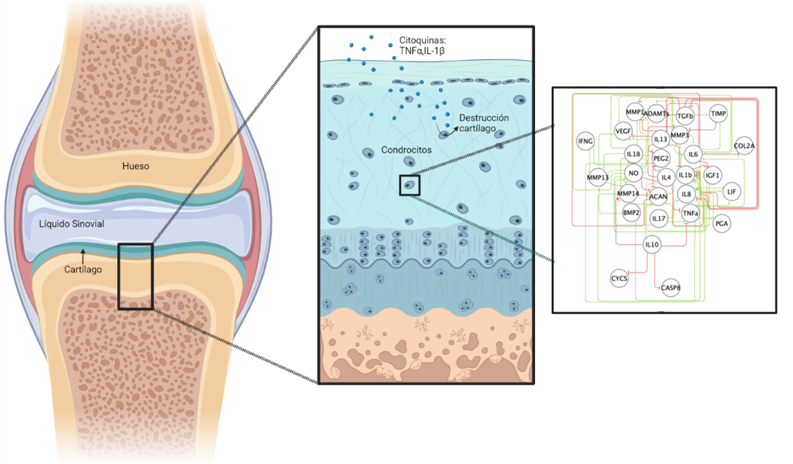
The Biomechanics and Mechanobiology area of UPF’s BCN MedTech research unit is developing a computational model of the regulation of proteins by chondrocytes, a type of cell found only in cartilaginous tissue. The initial results on the development, validation and usefulness of the model have been published in the journal Scientific Reports.
The Biomechanics and Mechanobiology area of UPF’s BCN MedTech research unit is developing a computational model of the regulation of proteins by chondrocytes, a type of cell found only in cartilaginous tissue. The initial results on the development, validation and usefulness of the model have been published in the journal Scientific Reports.
The dysregulation of the activity of articular cartilage chondrocytes is the basis of osteoarthritis, a degenerative disease affecting more than 3% of the world’s population whose incidence increases in general with age. The disease is also more prevalent among women. It is a debilitating disease whose most typical manifestations are chronic pain, functional alteration, and a reduction in people’s quality of life.
PhD researcher Maria Segarra-Queralt is the main author of the study and explains that “behind osteoarthritis there is a decompensation between the synthesis (anabolism) and the degradation (catabolism) of the components of the extracellular matrix by chondrocytes, but so far we do not properly understand how this process works”. Jérôme Noailly, co-director of the study together with Gemma Piella, explains that “it is very complicated to measure and anticipate the simultaneous effects of all the factors that affect the dynamic regulation of chondrocytes and are involved in osteoarthritis. For this reason, computational modelling has become increasingly important in the study of such pathologies and their treatments”.
The dysregulation of the activity of articular cartilage chondrocytes is the basis of osteoarthritis, a degenerative disease affecting more than 3% of the world's population whose incidence increases in general with age. The disease is also more prevalent among women.
Despite the hard work and progress made in regenerative medicine, there is no known mechanism to reverse the balance between synthesis and degradation, which affects the efficiency and reproducibility of possible regenerative treatments. For this reason, conventional treatments focus on trying to control the symptoms, but they do not cure and are not lasting. “So far, what we know is that cartilage does not regenerate itself and that is why we have to properly understand how this imbalance occurs”, continues Segarra-Queralt.
There are two theories as to the origin of this degradation: one considers that it is the biomechanical stimuli (due to trauma or caused by use) that lead to greater destruction of the cartilage and stimulation of the cells towards procatabolic activity. The other theory blames inflammation, expressed through a large presence of cytokines that increase the expression of enzymes that degrade cartilage. “Most likely, at some point both mechanisms work together”, adds Jordi Monfort, head of rheumatology at Hospital del Mar and co-author of the study. States of health with inflammatory processes, such as metabolic syndrome, are common among patients with osteoarthritis.
The research, in collaboration with researchers in systems biology, from the National Technical University of Athens, and in inflammation and cartilage, from the Hospital del Mar Medical Research Institute (IMIM) in Barcelona, analyses the interactions between the main inflammatory proteins and the enzymes responsible for degrading cartilage at the cell level using a network-based mathematical model. “We create around 1800 different models and what we present in the study is what best predicts (to 95% accuracy) the behaviour of the chondrocytes”.
They began by analysing the existing literature on these interactions and saw that there was a clear deviation towards catabolism. “In other words, these models could only give us information about the procatabolic activity of chondrocytes and did not take into account the ability to synthesize new cartilage”, which greatly limits the exploitation of the knowledge available to explore and understand the transitions between the biosynthetic processes that protect cartilage and the catabolic processes that degrade the tissue. For this reason they had to create a model that did take this synthesis into account.
To avoid bias in inputting data of the new variables, which is very common in computer modelling, they used in vitro experiments to calibrate the model parameters and validate the results.
“The model would be a first step towards the rapid screening of possible treatments suitable for a given person. In this way we could predict the best treatment for each patient, which will lower the financial cost”
In its current version, this model has already enabled better understanding treatment results for osteoarthritis through injections of autologous anti-inflammatory factors. It will also allow personalized predictions based on the data of each patient, including biological analysis of the synovial fluid of the joints. “The model would be a first step towards rapid screening of possible treatments suitable for a given person. In this way we could predict the best treatment for each patient, which will lower the financial cost”, Segarra-Queralt concludes.
In a second part of the study, Segarra-Queralt, Piella and Noailly are incorporating biomechanical stimuli into the model in order to understand how knee cartilage cells behave under certain mechanical stress situations. For this work, in October 2021 Segarra-Queralt received the best scientific contribution award, granted by the Spanish National Chapter of the European Society of Biomechanics (CAPESB).
The research was carried out with the support of the Catalan Government (scholarship 2020 FI_B 00680), Marie Skłodowska-Curie Actions (project Disc4All MSCA-2020-ITN-ETN GA: 955735) and of the Spanish Government (scholarship RYC-2018-8; project HOLOA-DPI2016-80283-C2-1/2-R).
