Mathematicians calculate the number of immune cells needed to thwart HIV progress
In the future, a model of this kind will enable scientists to carry out computer experiments for the development of vaccines and medicines for infectious diseases. The study, led by Russian researchers, involves Andreas Meyerhans, ICREA research professor at UPF.
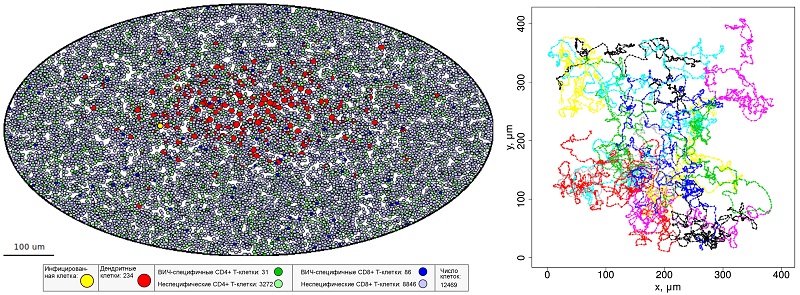
An international team of researchers have used the data on the mobility of immune T-cells to design a mathematical model in order to predict the number of them needed to be present in one lymph node to block further progress of Human Immunodeficiency Virus (HIV). The study has been led by Russian researchers at the Marchuk Institute of Numerical Mathematics of the Russian Academy of Sciences and involves Andreas Meyerhans, ICREA research professor at UPF.
In the future, a model of this kind will enable scientists to carry out computer experiments for the development of vaccines and medicines for infectious diseases. The results of the research has been published in Frontiers in Immunology and has been supported by Russian Science Foundation.
“This model enabled us to forecast how many HIV-destroying T-cells there should be so that these cells, considering their mobility, could stop the spread of the virus. Like any other model, ours ignores many factors but makes it possible to determine what we must take into account while working on a HIV vaccine”- said Gennady Bocharov, coordinator of the study.
The model enabled to forecast how many HIV-destroying T-cells there should be so that these cells, considering their mobility, could stop the spread of the virus.
Mathematical models are widely used by scientists to explore the functioning of many complicated systems – from global markets to oil and gas reserves to protein molecules. A computer model can provide a simulation of the functioning of human and animal immune systems. A single model of this kind would make it possible to predict the effect of medicines before clinical trials and conduct a thorough scrutiny of the course of the disease without additional laboratory experiments. Most models that exist in immunology focus on separate processes, rather than on a system as a whole. A study of the system as a whole requires taking into account biological effects on all levels: cells, organs and the organism as a whole.
Mathematical descriptions of the movement of immune cells in 3D spaces of organs and tissues should become the core of such an integrated model. Russian researchers have created a model of this kind for T-lymphocyte movements within a lymph node. For calculations they have selected a space in the so-called paracortical zone of a lymph node where a particularly large number of T-cells is concemtrated. The model is based on basic principles of physics. Each of the more than 12 thousand T-lymphocytes under scrutiny moves under the influence of forces that act on it in accordance with Newton’s Second Law: the product of speed by body mass equals the sum of the forces acting on the body. Mathematicians have calculated the parameters of these forces on the basis of the published results of immunological studies. The movement of each T-lymphocyte in the model depends both on its active internal mobility, and on its interactions with other cells, as well as on the friction force in the viscous medium of a lymph node.
The movement of each T-lymphocyte in the model depends both on its active internal mobility, and on its interactions with other cells, as well as on the friction force in the viscous medium of a lymph node.
Scientists have resorted to the model to establish how lymphocyte motility affects their ability to spot and destroy HIV-infected cells. Studies of experimental vaccines demonstrate that the effectiveness of immune response to the virus depends on the number of "tuned", that is, effector antigen-specific to HIV, CD8 + T-lymphocytes in the lymph nodes. But until recently scientists could not determine how many of these lymphocytes there should be, so that all infected cells were detected.
The mathematical model has made it possible to reproduce the spread of infection in silico, that is, in a computer experiment. Between 18 and 24 hours pass before HIV-infected cells release viral particles. As it turned out, if 5% of all CD8+ T-lymphocytes in the lymph node are effector, antigen-specific to HIV and their mobility is normal, the infected cells will be detected in less than 18 hours.
However, viral infection reduces lymphocyte mobility due to the growth of connective tissue in the lymph nodes. And if this mobility is reduced by half, there is more than 50% likelihood that CD8 + T-lymphocytes will not have time to stop the spread of the virus. This means that future HIV vaccines should form more than 5% of antigen-specific CD8+ T-lymphocytes and guarantee their normal mobility.
In the future, the model can be adapted for the study of other infectious diseases. The mechanism of cell migration under the influence of forces that ensure their autonomous movement, and the mechanism of intercellular interaction are quite universal.
“In the future, our model can be adapted for the study of other infectious diseases. The mechanism of cell migration under the influence of forces that ensure their autonomous movement, and the mechanism of intercellular interaction are quite universal, - said Gennady Bocharov. - “But each specific infection will require some parameters of the model to be adjusted, using appropriate experimental and clinical data. In addition to infectious diseases, the model can be useful in the study of malignant tumors."
Participating in the research were also scientists from the Moscow Institute of Physics and Technology, Peoples’ Friendship University of Russia, the Institute of Problems of Mechanical Engineering of the Russian Academy of Sciences, and Uppsala University (Sweden).
Reference article:
Grebennikov Dmitry, Bouchnita Anass, Volpert Vitaly, Bessonov Nikolay, Meyerhans Andreas, Bocharov Gennady. Spatial Lymphocyte Dynamics in Lymph Nodes Predicts the Cytotoxic T Cell Frequency Needed for HIV Infection Control. Frontiers in Immunology, June 2019. DOI: 10.3389/fimmu.2019.01213.
