Critical events during virus infection fate regulation
An international study led by the Infection Biology Group at UPF shows a communication axis that is part of a functional adaptation to chronic viral infections.
Infections with pathogenic viruses are in most cases either resolved by the immune system (acute infections) or become chronic and thus remain present in a host for ever. Examples of this latter case are infections with the Human Immunodeficiency Virus (HIV) or the Hepatitis B and C viruses (HBV, HCV).
Scientists led by Andreas Meyerhans together with other researchers from Spain, Russia, Switzerland, and Japan have now studied acute and chronic virus infections from a Systems Biology approach. Their results appear in an article published today in Genome Research.
The development of an acute or chronic viral infection is the result of a timely coordinated response of the immune system to the invading virus. A chronic infection arises when immune effector mechanisms are shut down to avoid immunopathology because the simultaneous presence of widespread virus infection and our strong cellular responses can induce massive cell and tissue destruction, which may threaten the life of the infected host. “How an infected host senses such a threat and how it adapts its immune response accordingly is not completely understood”, explains Jordi Argilaguet, first author of the study.
They have studied acute and chronic virus infections from a Systems Biology approach.
“While most previous immunological studies of virus-host interactions focus on individual cell subsets or specific pathways, we decided to obtain a more global view of the underlying processes”, he adds. They used the lymphocytic choriomeningitis virus (LCMV) mouse model system. It allows establishing either an acute or a chronic infection and displays immunological features that resemble those of HIV or Hepatitis B or C virus infections in humans. In their experiments, spleen-derived transcriptomes were obtained from infected mice at different time-points after infection and analysed.
Bioinformatic analyses performed by Anna Esteve-Codina, from the group of Simon Heath (CNAG-CRG), allowed the identification of clusters of highly co-expressed genes (modules) that represent biological processes regulated in the host in response to the infection. Importantly, several modules appeared to be specific to acute or chronic infection and therefore represent specific features of the infection fate.
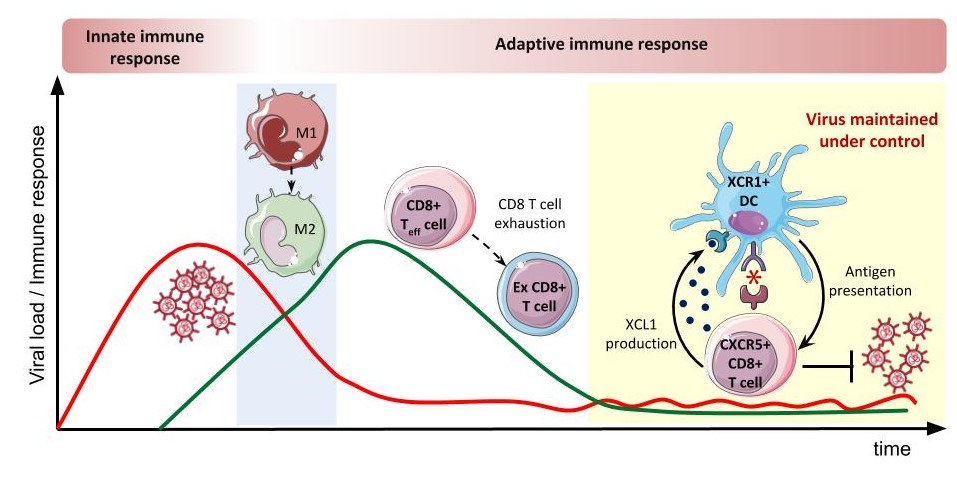
One of the key mechanisms by which immune mechanisms are shut down to avoid immunopathology is T cell exhaustion. A T cell is a type of lymphocyte that plays a central role in the immune response, thanks to its ability to specifically recognize foreign antigens and respond to their presence. Having been exposed to the same antigen for a long period of time, they must be switched off to avoid excessive damage in healthy tissues.
Researchers identified clusters of highly co-expressed genes that represent biological processes regulated in the host in response to the infection.
According to Andreas Meyerhans, ICREA research professor at UPF “in this study, we demonstrate an early attenuation of inflammatory monocytic cells, which are a type of white blood cells, prior to T cell exhaustion”. This suggests that attenuation of these inflammatory monocytes helps to prevent the development of fibrosis -which develops as a consequence of inflammation- in the spleens of chronically infected animals.
The authors demonstrate the involvement of a communication axis (XCL1-XCR1) between T cells and cross-presenting dendritic cells, which present extracellular antigens to the T cells, in virus containment during chronic infection. Despite the various suppressive mechanisms induced during chronic infection, effector T cell exhaustion is only partial and some T cell functionality remains that restrains the expansion of the persisting virus. Researchers show here that this communication axis is critical to maintaining such effector T cell responses.
Further studies are now ongoing in the group to exploit these observations for host benefit. Enforcing the XCL1-XCR1 axis in an immunotherapeutic intervention may improve the immune response. “Translating the study to humans targeting these immune control mechanisms seems promising in the quest to functionally cure chronic infections like HIV”, concludes Meyerhans.
Reference article:
Jordi Argilaguet, Mireia Pedragosa, Anna Esteve-Codina, Graciela Riera, Eric Vidal, Cristina Peligero-Cruz, Valentina Casella, David Andreu, Tsuneyasu Kaisho, Gennady Bocharov, Burkhard Ludewig, Simon Heath, and Andreas Meyerhans. Systems analysis reveals complex biological processes during virus infection fate decisions. Genome Research, May 2019. DOI: 10.1101/gr.241372.118.
